How does reverse osmosis work?

Reverse osmosis is a system for purifying water with a very fine filtering system that allows practically only water molecules to pass through. To date, it is the only ideal process for effectively purifying your tap water for consumption. It is not a coincidence that NASA is currently using it on board the ISS!1

History
The term "osmosis" stems from the Greek ὠσμός meaning "push".3
Reverse osmosis was developed around 1959 by Sidney Loeb at the University of California. It was industrialised in the 1970s. 4
How it works
The principle of osmosis is the spontaneous passage of a diluted liquid to a liquid with a higher salt concentration through a semipermeable membrane (like a sieve). The liquid passes through the membrane, whereas the dissolved solids don’t.3
In the case of reverse osmosis, this phenomenon is forced to reverse itself by exerting pressure on the liquid with a higher concentration of residues. This will diffuse practically only the water molecules on the other side, filtering it to a tenth of a nanometre (1/10,000,000th of a millimetre). This process happens completely naturally without the addition of any chemicals.
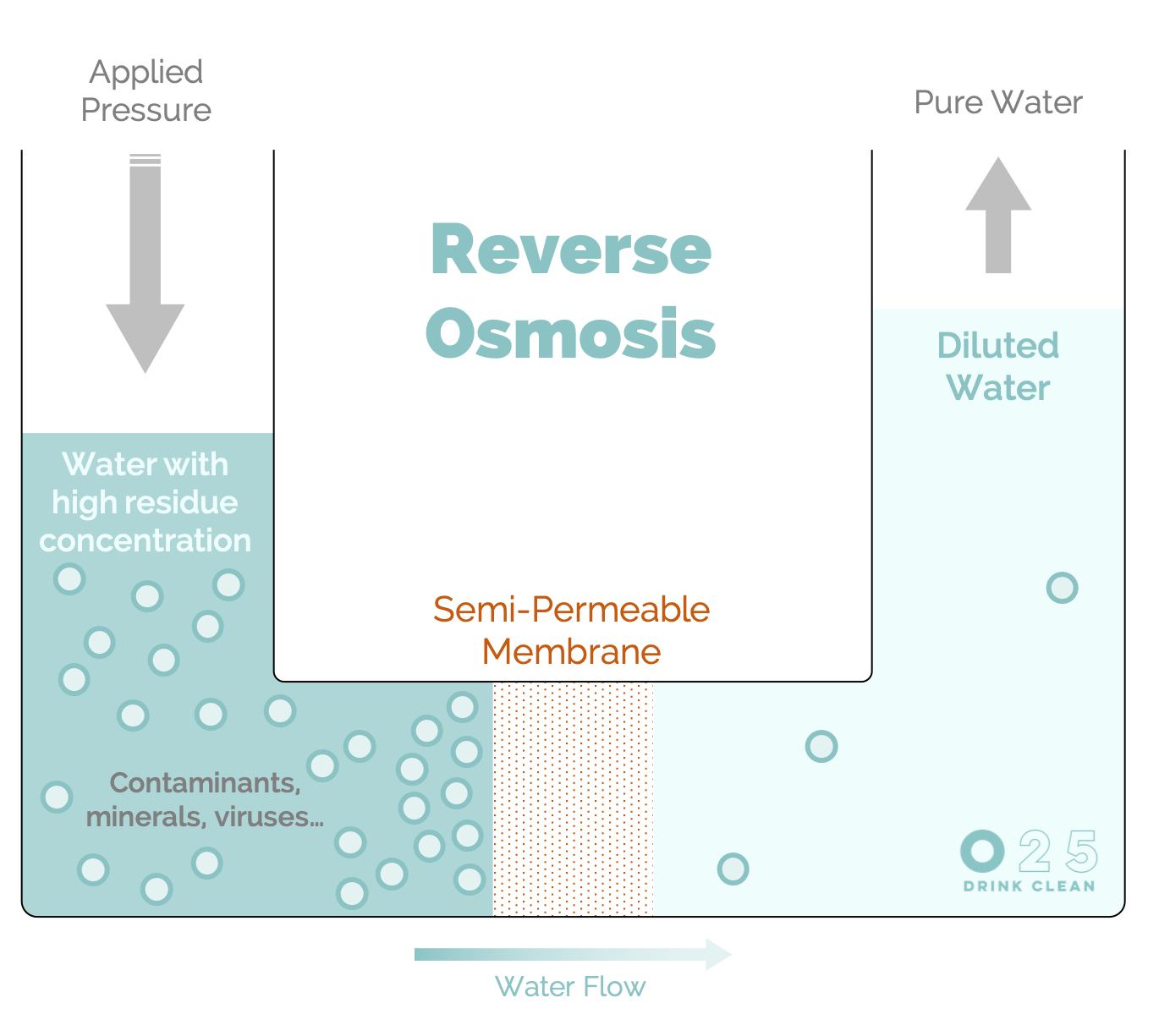
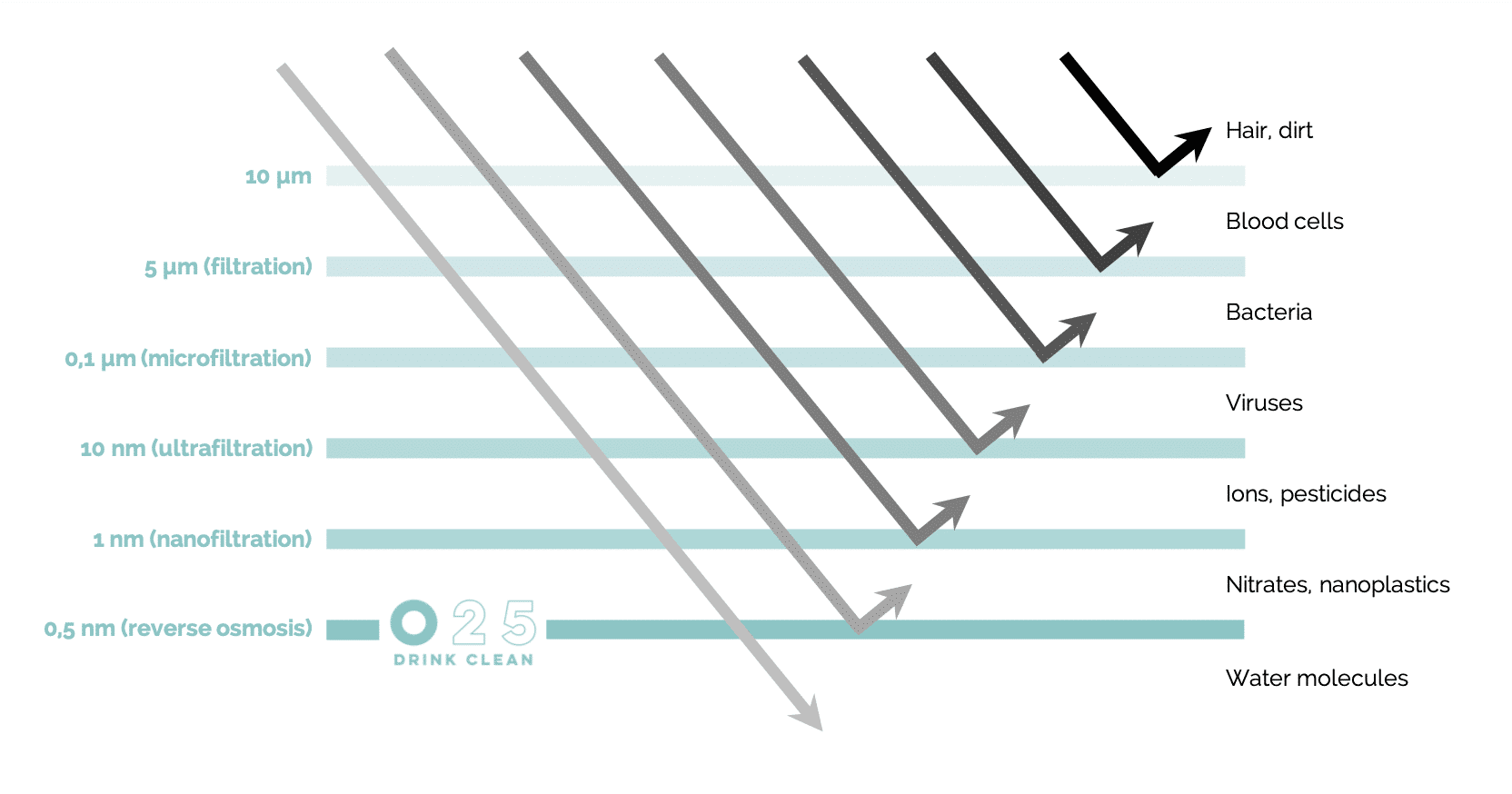
Heavy metals, microplastics, bacteria, viruses, drug residues, pesticides and nitrates... Practically nothing passes through the membrane. The O25 osmosis system guarantees a maximum of 25 mg of dry residues per litre, a reduction of more than 90%!
Efficiency
The last couple of years, the efficiency of an osmosis system has improved significantly. From a ratio of 2 to 6 litres of water used for 1 litre of purified water, the conversion rate of domestic osmosis systems has decreased to 1 litre rejected for 1 purified litre. Depending on your installation, this water can be recovered for other purposes (e.g. watering, sanitary, cistern, etc.).
While it is true that some water is lost, this choice is more interesting than water in plastic bottles. Their production requires 4 litres of water. This is even without mentioning the carbon footprint and the waste generated!5
The result: soft, light water that is pleasant to drink. This improves the well-being and also has a positive impact on the environment by reducing the quantity of purchased bottles.
______________
1 https://spinoff.nasa.gov/Spinoff2019/ps_5.html
3 https://www.universalis.fr/encyclopedie/membranes-transferts
4Loeb, S., 1981. The Loeb-Sourirajan Membrane: How It Came About, In: Turbak, A. ed. Synthetic Membranes: Volume I Desalination, Washington, DC: American Chemical Society, pp. 1-9
5 https://foodprint.org/blog/plastic-water-bottle/



